
Clinical and Procedural Guidelines for the Gastrointestinal Endoscopy Unit during the Covid-19 Pandemic

The COVID-19 disease is declared a national health emergency and a pandemic. These guidelines are written based on the World Health Organization (WHO) warning system and can be updated based on the developing public health situation. The contents and recommendations in this guide are interpretations of the best-published information by experts.
These guidelines are intended to supplement information but do not replace relevant recommendations or policies from institutions related to infectious diseases. Please consider these recommendations to be applied to your unit in accordance with your resources and infection prevention strategies.
Current Condition of COVID-19 and Risk of Transmission
• WHO estimates that the mortality rate until March 3, 2020, is 3.4-8%
• The mechanisms for transmitting COVID-19 are as follows:
“COVID-19 is transmitted through droplets and surrounding objects contaminated with the virus (fomites) when in close contact with someone who has been infected. There have been no reports regarding airborne transmission as the main mechanism of transmission of this virus and there is no valid evidence of this. However, it remains a consideration for some procedures in health facilities that can produce aerosols. Several case report studies suggest that the virus is identifiable and viable in stool samples of infected patients. However, the fecal-oral route is not suspected to be a transmission mechanism for COVID-19; Its role and significance for COVID-19 is still in the process of being searched
“We have isolated the SARS COV2 virus from feces which confirms the release of virions in the gastrointestinal tract. Therefore, fecal-oral transmission can be an additional route for viral spread ”. 2
• Most of the patients had complaints in the form of fever (98.6%), fatigue (69.6%), and dry cough (59.4%), and other constitutional symptoms. However, many patients also experienced gastrointestinal complaints such as diarrhea (10.1%) and nausea (10.1%). 3
• The potential mode of transmission during gastrointestinal endoscopy can occur through respiratory secretions during upper gastrointestinal endoscopy (esophago duodenoscopy) and exposure to feces during colonoscopy (inhalation, conjunctival splash, and direct touch) .4,5
• To minimize the risk of droplet inhalation, it is recommended to maintain a minimum distance of
6 (six) feet or 2 (two) meters from someone who is potentially infected
• Some experts estimate the incubation period for COVID-19 to be around 1-14 days. Most of them lasted about 5 (five) days
To prevent transmission of COVID-19 in the gastrointestinal endoscopy unit, some of the recommended steps to be implemented and adhered to are as follows:
1). Patient Selection and Screening
• If the infection rate is still high, limit measures for emergency procedures such as gastrointestinal bleeding, foreign bodies, acute cholangitis, tumors requiring immediate histopathological diagnosis, or access to nutrition.
• Elective procedures are strongly advised to be postponed until the SARS COV2 crisis is over
• All patients should be screened for travel history, contact with confirmed cases, and symptoms that suggest COVID-19. If there is, then the patient is asked to postpone the procedure for at least 14 (fourteen) days. Patients with fever and patients with fatigue/malaise, cough, and/or diarrhea should be transferred to the emergency department (IGD) for further management. If no significant travel history is found (for example Chinese nationality and foreigners from countries that have reported cases of COVID-19) then action can be taken on the patient. A screening/screening form containing information on the patient’s travel history, risk of potential exposure and presence of symptoms, must be completed by the patient and/or interviewer before the procedure is carried out
• In addition to the routine informed consent form, ensure that the patient or family member has signed an “Informed consent for gastrointestinal endoscopy during the SARS-COV2 crisis.” 4
• It is recommended to screen with a chest X-ray or CT chest plus a rapid SARS-CoV2 examination or with a PCR swab adapted to existing facilities.
• All patients and relatives (only one adult who is responsible for the patient) must be available for temperature checks and at least must wear a surgical mask before entering the endoscopy unit
Table 1. Classification of Types of Endoscopy Actions

2. Endoscopy Room
● Limit the number of operational endoscope spaces to maintain supplies of personal protective equipment. The procedure is performed by a trained endoscopist (Consultant) to limit the time and exposure of the procedure (for example by limiting the involvement of fellow or trainees) .6,7
● For highly suspected or confirmed cases, the procedure should be carried out in a negative pressure room. 6.8
● Clean the entire surface of the endoscope chamber thoroughly after each procedure is completed. Change all beds and pillows after each procedure. It is recommended to disinfect the room walls, tables, or endoscope equipment with chlorine spray. Every day the floor can be cleaned using a detergent that contains chlorine. 8
● Endoscopy report preparation can be done in a separate clean room and supervised by an endoscopist. 6
3. Protection of Staff / Health Workers
• Any staff who has complaints of fever, fatigue, dry cough, diarrhea or a history of contact with a patient infected with COVID-19 must be identified and referred to an infection control committee for appropriate treatment
• Temperature checks shall be carried out with a non-contact thermometer for all persons at the start of the working day and before entering the endoscope unit
• Staff are advised to change into the clothes provided by the hospital when entering the endoscopy unit. Previously worn clothing (clothes from home) should be stored and then reused when staff leave the endoscope unit (at the end of the working day)
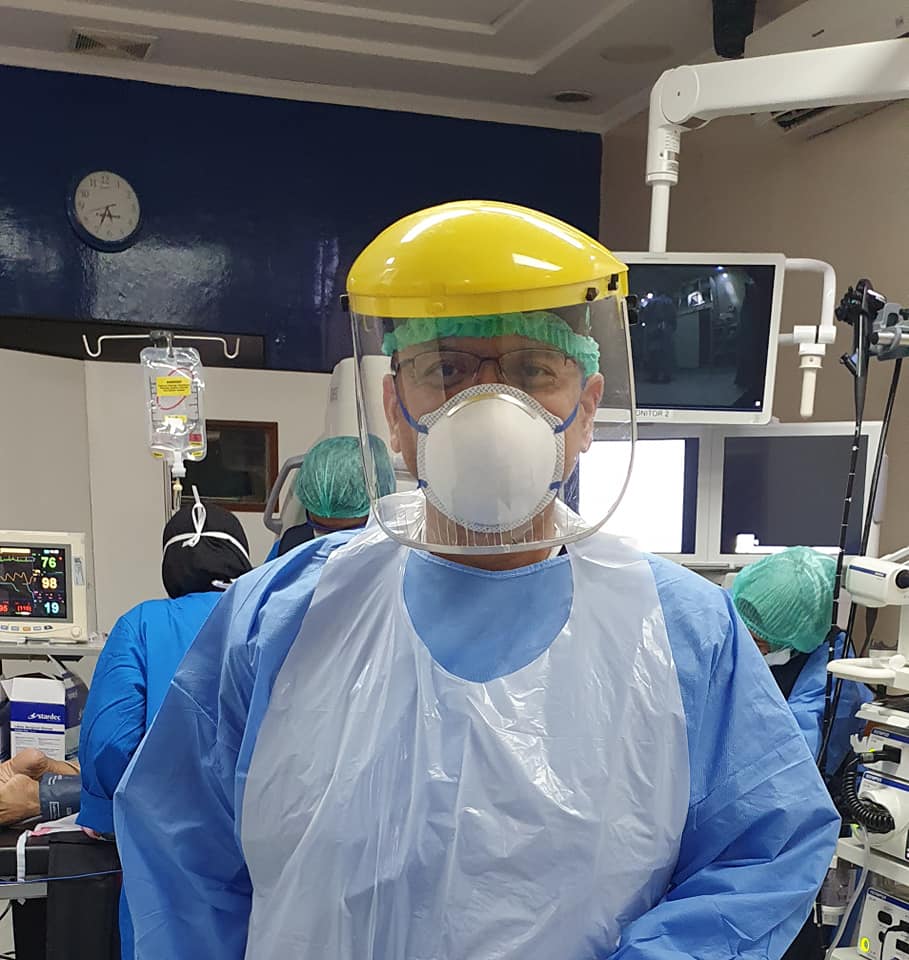
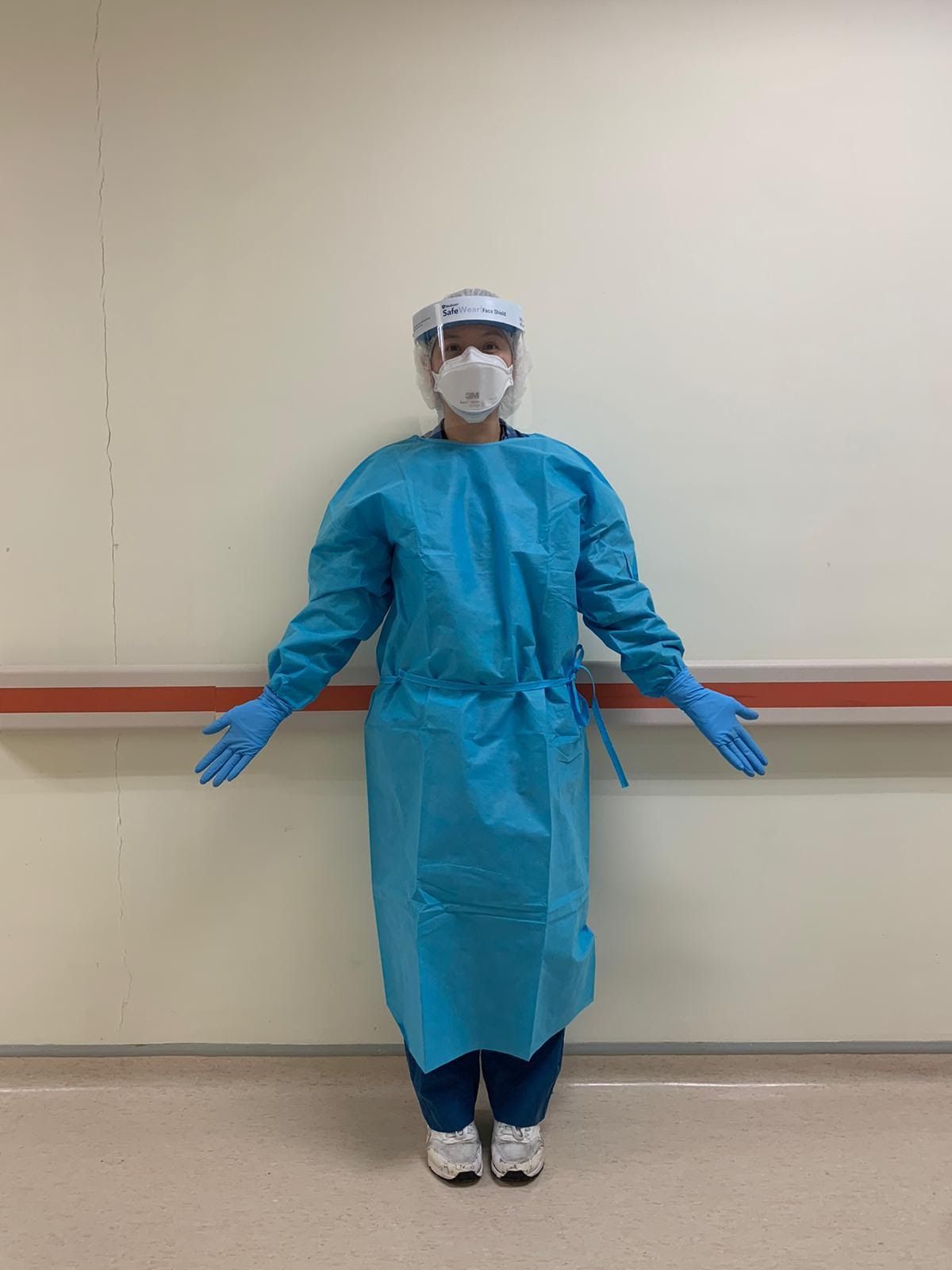
• For patients who are confirmed negative or at low risk, protection at Biosafety Level II is required for staff who come into direct contact with the patient (Endoscopist, Anesthetist, Nurse, and Assistant): disposable waterproof gown, N95 / FFP2 / FFP3 mask or surgical masks, goggles, hats and shoe covers during the procedure. 6,7,8
• For suspected high risk or confirmed cases, protection is required at Biosafety Level III for staff who have direct contact with the patient (Endoscopist, Anesthetist, Nurse, and Assistant): waterproof gown, full face protection / full face shield or mask N95 with goggles and boots, double-layer gloves and negative pressure air/chamber purifier. 6,7,8
• Protection at Biosafety Level III is required when performing tracheal intubation, airway care, and sputum suction even in patients with suspected low or unconfirmed risk. 3,8
• Patient receiving staff must also be protected by at least wearing a surgical mask.3,8 After all procedures are carried out, all personal protective equipment (PPE) must be removed and disposed of properly, namely in infectious trash cans and following applicable institutional policies. Hands and exposed areas should be washed and disinfected immediately. Surgical masks are required in all areas of the unit.
• The shower/sink area should be available and easily accessible in case of contact or contamination
4. Waiting Room and Recovery Area
• The waiting room must have sufficient space, at least 3-6 feet or 1-2 meters between one patient and another patient to prevent droplet inhalation
• The recovery area must provide sufficient privacy and space, at least 6 (six) feet or 2 (two) meters between one patient and another to avoid droplet inhalation and for monitoring and treatment
5. Accessory Scope / Processing and Disinfection
• For low risk, reprocessing is carried out after implementing universal precautionary standards
• For high risk or confirmed cases, 2 (two) reprocesses are required
• Accessories must be disposed of immediately in the appropriate infectious trash and in accordance with applicable institutional policies
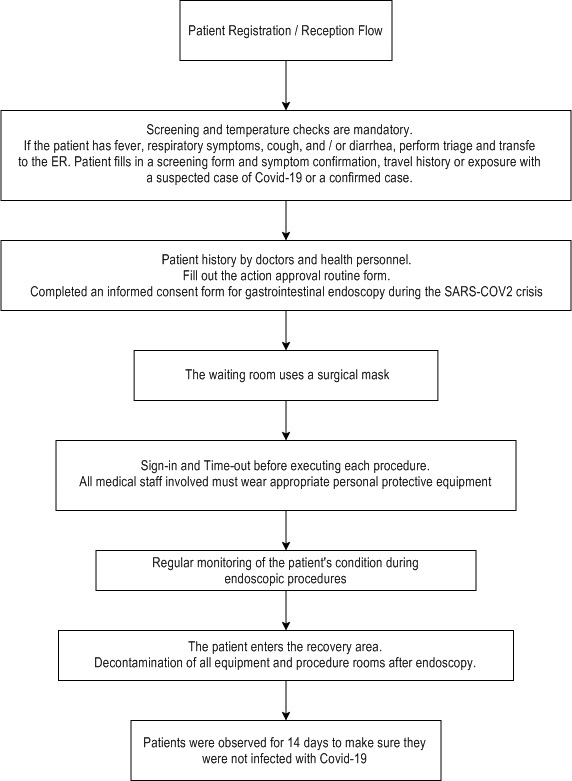
Diagnostic and Therapeutic Workflow in the Endoscopy Unit during the Crisis SARS COV2
Recommendations for the Use of PPE
Reference
1. Rio C, Malani P. COVID-19-new insights on a rapidly changing epidemic. JAMA. 2020; 323 (14): 1339-40. DOI: 10.1001 / jama.2020.3072
2. Chiu PW, Ng SC, Inoue H, Reddy DN, HU EL, Cho JY, et al. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE-COVID statements). Gut. 2020; 69 (6): 991-6. DOI: 10.1136 / gutjnl-2020-321185
3. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020; 158 (6): 1831–3.e3. DOI: 10.1053 / j.gastro.2020.02.055
4. Zhang Yafei, Zhang Xiaodan, Liu L, Wang Hongling, Zhao Qiu. Suggestions for infection prevention and control in digestive endoscopy during the current 2019-nCoV pneumonia outbreak in Wuhan, Hubei Province, China. Endoscopy. 2020; 52 (4): 312–14
5. Report of WHO-China. Joint mission on coronavirus disease 2019 (COVID-19)
6.Sultan S, Lim J, Altayar O, Davitkov P, Feuerstein J, Siddique S, et al. The AGA institute rapid recommendations for gastrointestinal procedures during the COVID-19 pandemic. Gastroenterology. 2020; S0016-5085 (20) 30458-3. DOI 10.1053 / j.gastro.2020.03.072
7. Soetikno R, Teoh AYB, Kaltenbach T, Lau JYW, Asokkumar R, et al. Considerations in performing endoscopy during the COVID-19 pandemic. Gastrointest Endosc. 2020; 92 (1): 176-83. DOI: 10.1016 / j.gie.2020.03.3758
8. Gralnek IM, Hassan C, Beilenhoff U, Antonelli G, Ebigbo A, et al. ESGE and ESGENA position statement on gastrointestinal endoscopy and the COVID-19 pandemic. Endoscopy 2020; 52 (6): 483-90. DOI: 10.1055 / a-1155-6229

 Users Today : 654
Users Today : 654 Total views : 2234335
Total views : 2234335 Who's Online : 5
Who's Online : 5